Person with PH1
*Alnylam is proud to feature real patients in our advertising. Patients may or may not be on an Alnylam therapy.
ILLUMINATE: Pivotal Clinical Trials Evaluating OXLUMO® (lumasiran)
ILLUMINATE-A: The largest interventional study in children and adults with PH11
ILLUMINATE-A is an international, multicenter, phase 3, randomized, double-blind, placebo-controlled trial including adults and children ≥6 years with eGFR ≥30 mL/min/1.73 m2 (N=39)1,2
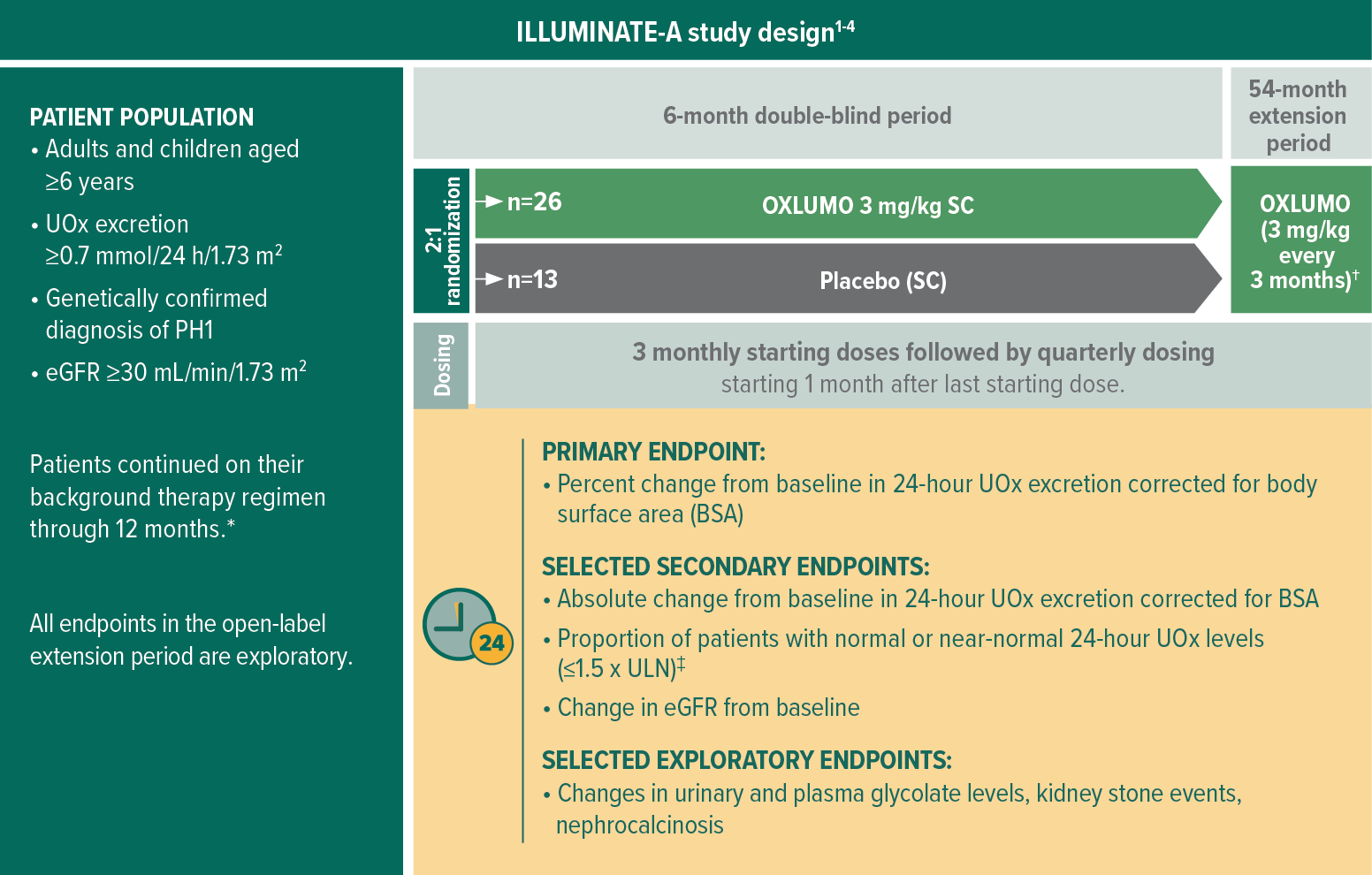
Selected baseline characteristics1,2:
- Mean age at first dose: 15 years (range: 6 to 61 years)
- Mean 24-hour UOx level corrected for BSA: 163.8 ± 55.8 mg
(1.82 ± 0.62 mmol) - Pyridoxine (vitamin B6) use: 56%
- eGFR: ≥90 mL/min/1.73 m2 (33%); 60 to <90 mL/min/1.73 m2 (49%); 30 to <60 mL/min/1.73 m2 (18%)
eGFR, estimated glomerular filtration rate; SC, subcutaneous; ULN, upper limit of normal; UOx, urinary oxalate.
Including hyperhydration, crystallization inhibitors, and/or pyridoxine, after which they can adjust per the recommendations of their treating physician.2
Patients randomized to placebo received starting doses of 3 mg/kg OXLUMO at months 6, 7, and 8; patients randomized to OXLUMO received an ongoing dose of 3 mg/kg OXLUMO at month 6 and placebo at months 7 and 8. At month 9, all patients started receiving OXLUMO every 3 months.5
1.5 x ULN, ≤69.4 mg/24 h/1.73 m2 (≤0.771 mmol/24 h/1.73 m2); ULN, ≤46.3 mg/24 h/1.73 m2 (≤0.514 mmol/24 h/1.73 m2).3
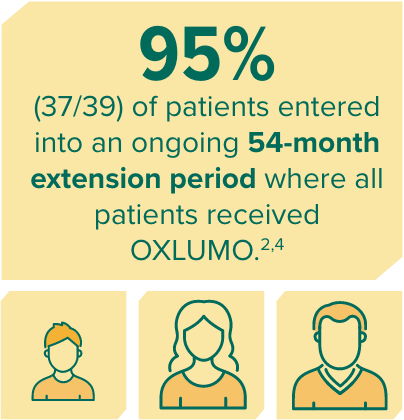
In ILLUMINATE-A, treatment with OXLUMO® (lumasiran) led to rapid, powerful, and sustained reduction in 24-hour UOx1,4
Rapid onset of reduction in UOx was observed within 1 month, with maximal reduction reached by month 2, and sustained through 36 months in patients initially treated with OXLUMO.1,4
24-hour UOx excretion over time*†1,4-6
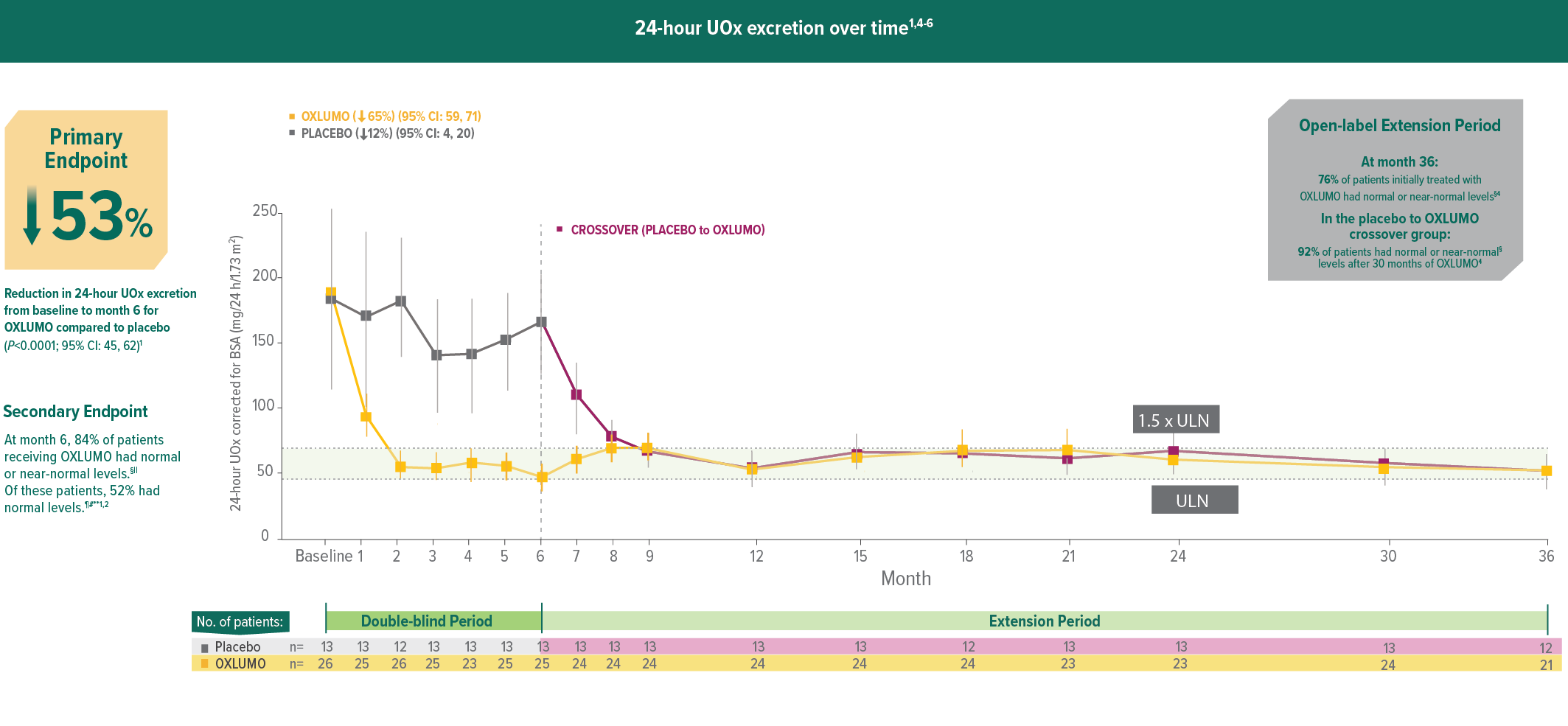
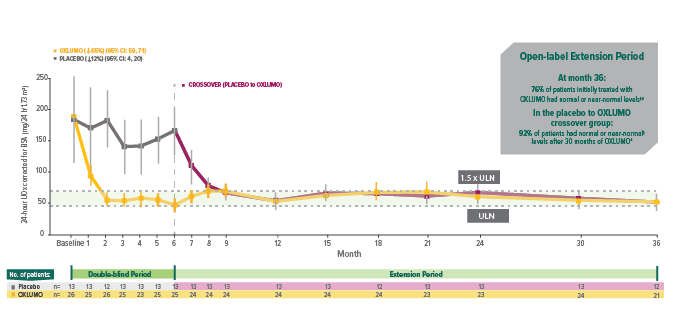
Mean and SEM of actual observed values.6
Assessments were conducted monthly until month 9; thereafter, assessments were conducted every 3 months until month 24.7
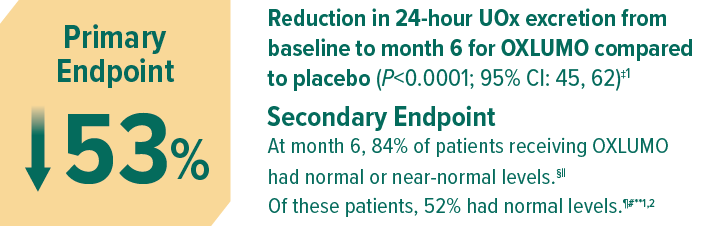
Least squares (LS) mean reduction averaged over months 3 through 6, corrected for BSA.1
Normal or near normal (at or below 1.5 x ULN): ≤69.4 mg (≤0.771 mmol)/24 h/1.73 m2.2,7
95% CI: 64, 95; P<0.001 vs placebo.
Normal (at or below ULN): ≤46.3 mg (≤0.514 mmol)/24 h/1.73 m2.2
95% CI: 31, 72; P=0.001 vs placebo.
The proportion of patients with levels above normal but below near-normal (32%) was not defined as an endpoint. Near-normal: >46.3 and ≤69.4 mg (>0.514 and ≤0.771 mmol)/24 h/1.73 m2.
In ILLUMINATE-B, evaluating OXLUMO® (lumasiran) in infants and young children with PH11
The multicenter, phase 3, single-arm, open-label trial includes patients aged <6 years and eGFR >45 mL/min/1.73 m2 (normal serum creatinine was used for patients aged <12 months*) (N=18)1
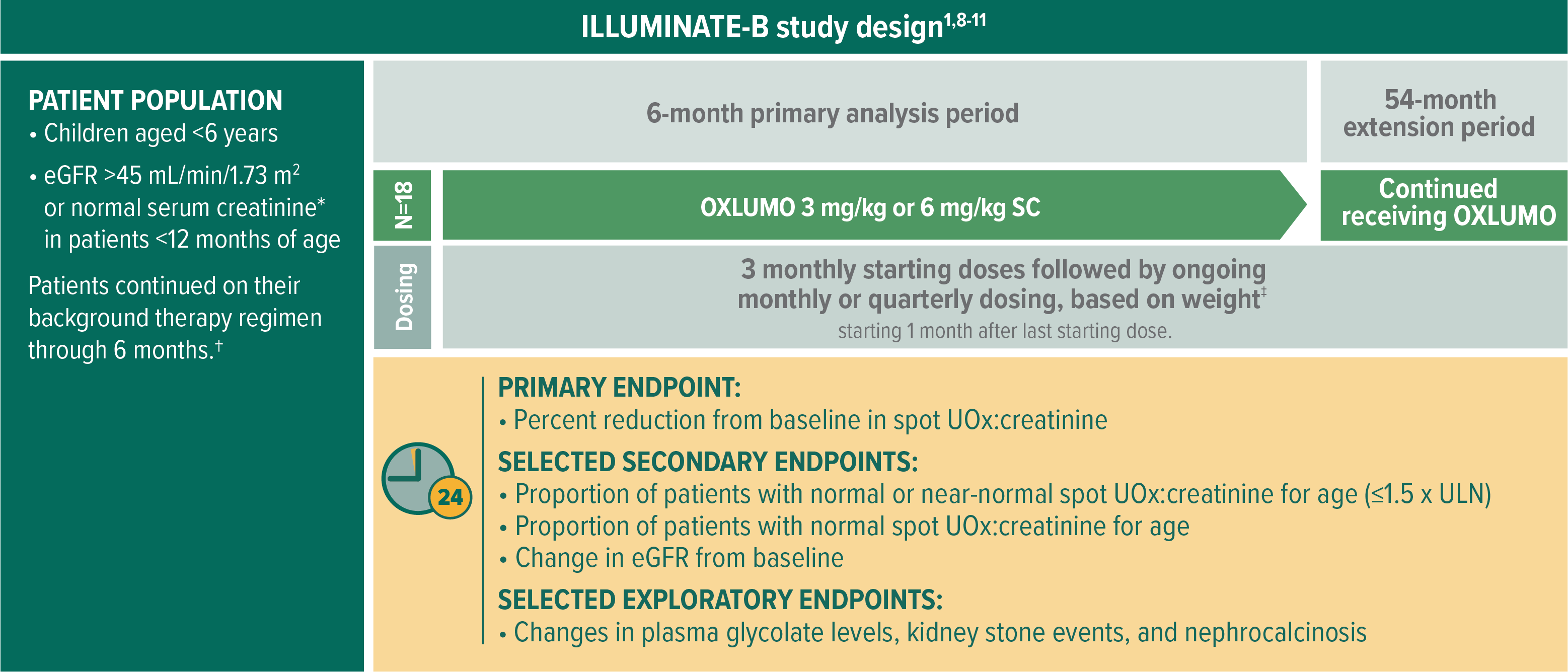
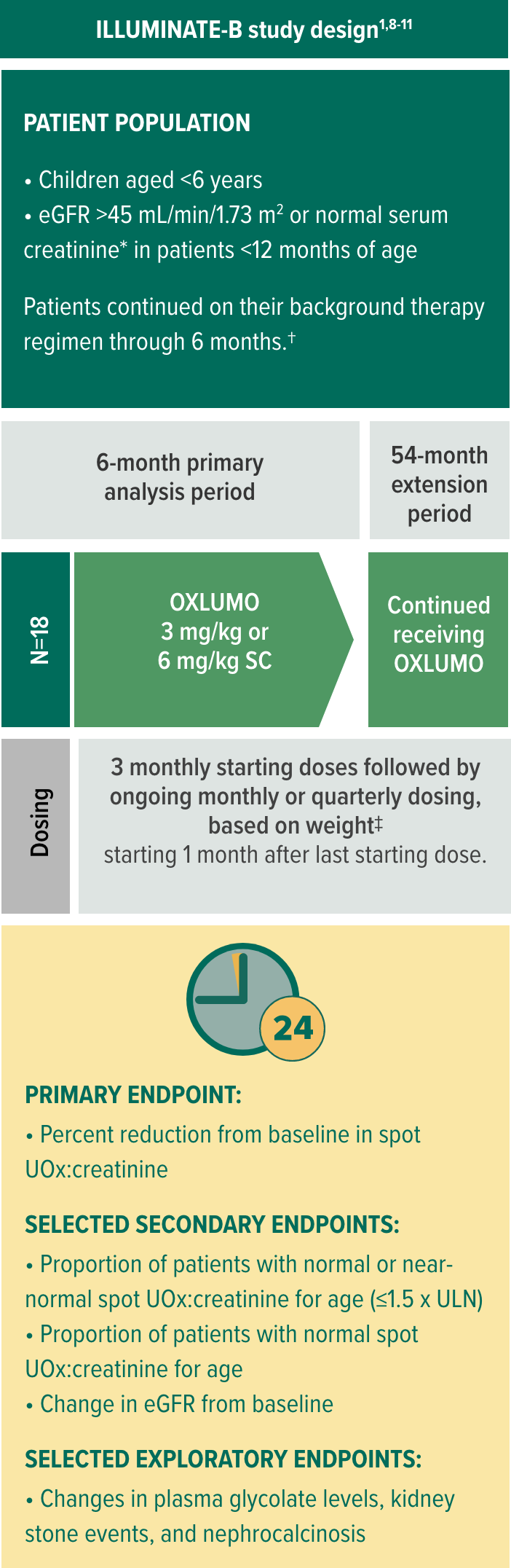

Selected baseline characteristics1,8,10:
- Median age at first dose: 51 months (range: 4 to 74 months)
- Median spot UOx:creatinine ratio at baseline: 0.37 mg/mg (0.47 mmol/mmol), range: 0.13-1.36 mg/mg (0.17-1.71 mmol/mmol)
- At baseline, the median eGFR was 111 mL/min/1.73 m2 (range: 65-174 mL/min/1.73 m2)
Age-dependent ULN.8
Including hyperhydration, crystallization inhibitors, and/or pyridoxine therapy.8
Patients <10 kg received starting doses 6.0 mg/kg monthly for 3 months and then ongoing doses 3.0 mg/kg monthly; patients ≥10 to <20 kg received starting doses 6.0 mg/kg monthly for 3 months and then ongoing doses 6.0 mg/kg every 3 months; patients ≥20 kg received starting doses 3.0 mg/kg monthly for 3 months and then ongoing doses 3.0 mg/kg every 3 months.8
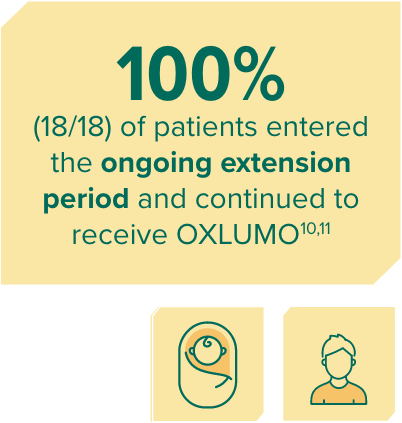
In ILLUMINATE-B, patients treated with OXLUMO demonstrated reduction in UOx at month 61
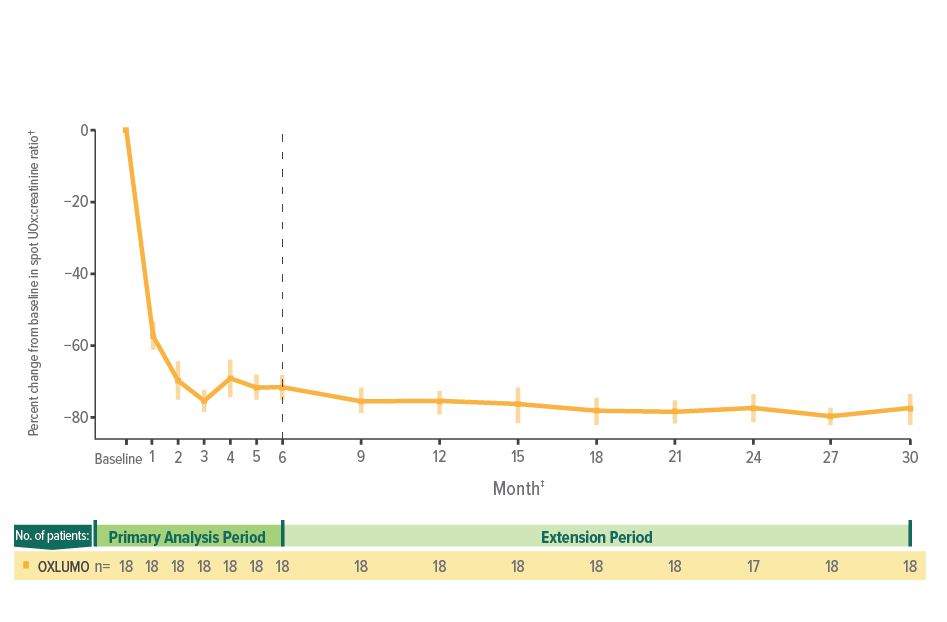
Rapid reduction in spot UOx:creatinine through 6 months, sustained through 30 months1,10
Spot UOx:creatinine over time1,10
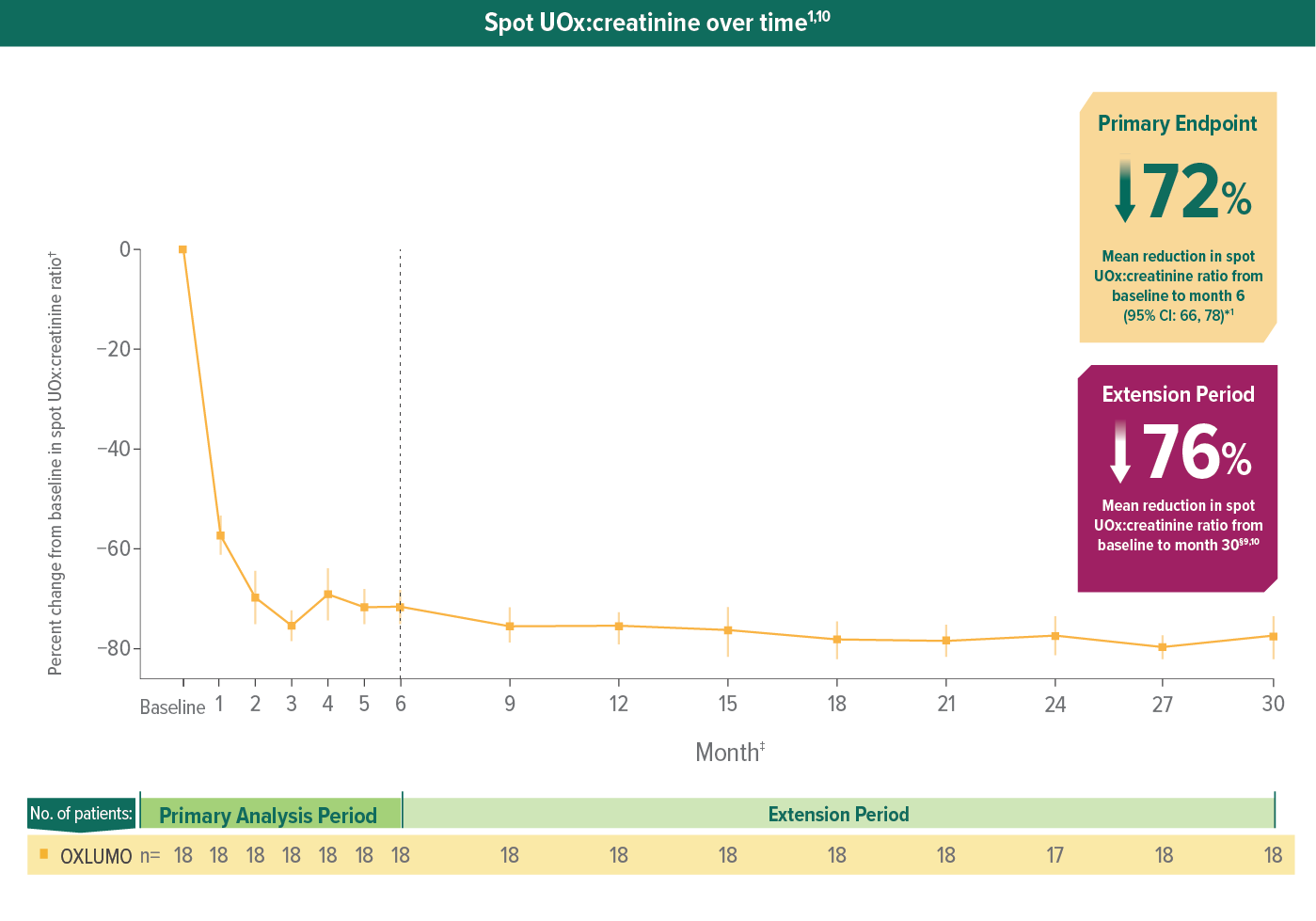
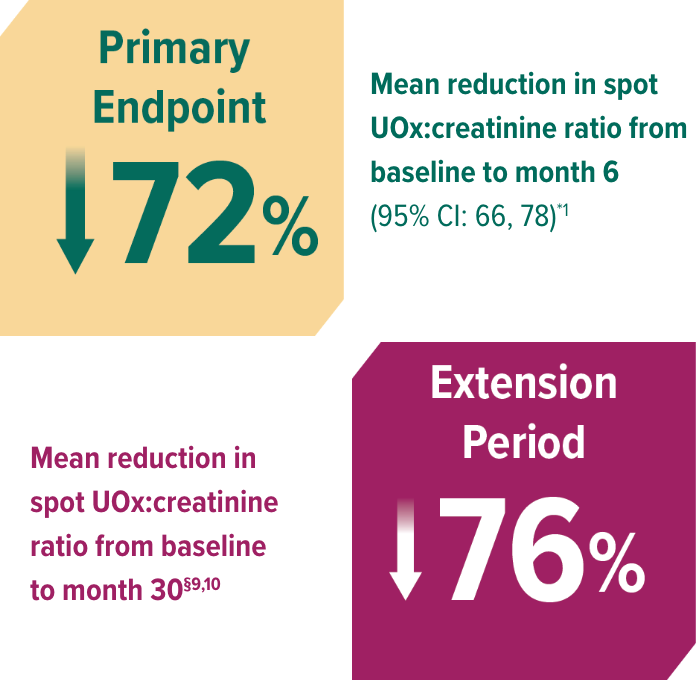
LS mean reduction averaged over months 3 through 6.9
Mean and SEM of percent change.10
After month 6, samples were only collected every 3 months for patients in ≥10 to <20 kg and ≥20 kg weight groups.
For patients in the <10 kg weight group, pharmacodynamic collections were optional at nonquarterly months.6
Mean spot UOx:creatinine ratio at month 30: 0.11 mmol/mmol.10
In ILLUMINATE-C, evaluating OXLUMO in more severely affected patients1,12
ILLUMINATE-C is a multicenter, phase 3, single-arm, open-label trial in patients of all ages with advanced kidney disease (N=21)1,12
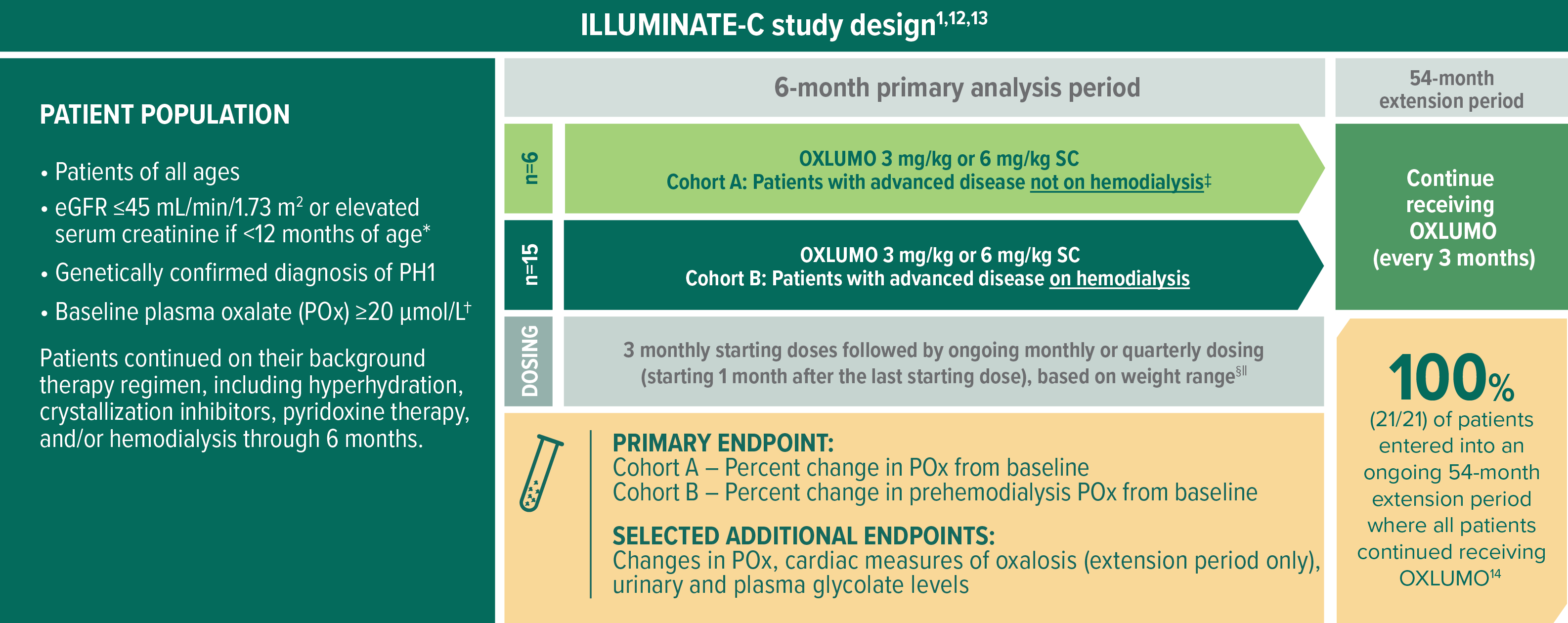
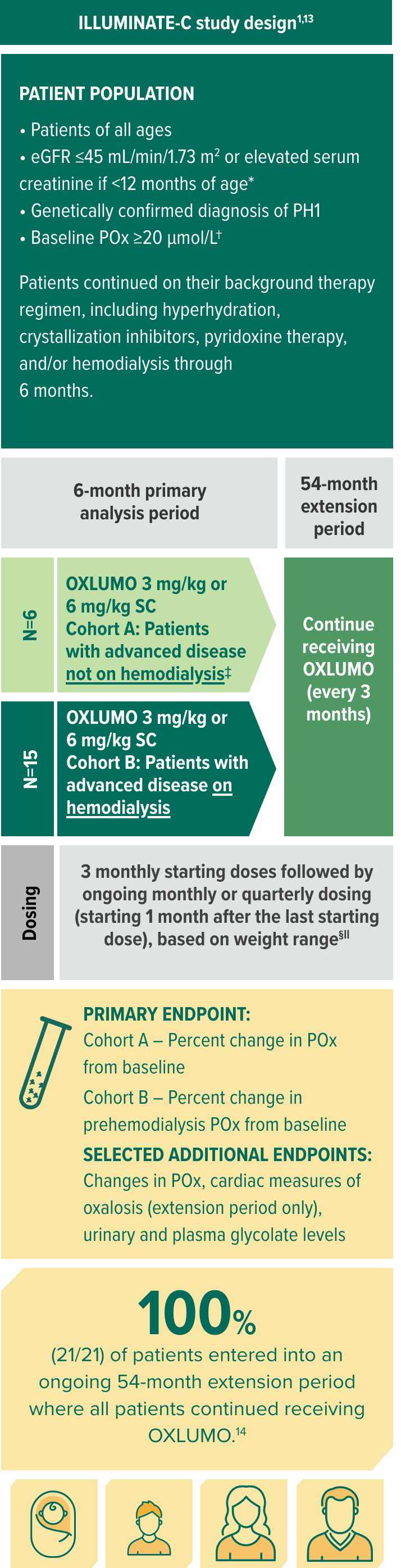
Selected baseline characteristics1:
- Median age at first dose: 9 years (range, 0 to 59 years)
- Median POx at baseline: Cohort A, 58 μmol/L; Cohort B, 104 μmol/L (prehemodialysis)
- Mean (95% CI) POx at baseline: Cohort A, 65 μmol/L (21, 108); Cohort B (prehemodialysis), 108 μmol/L (92, 125)
- Mean (SD) eGFR at baseline: Cohort A, 19.8 (9.6) mL/min/1.73 m2; Cohort B, not applicable
Above ULN for age.12
ULN=12.11 μmol/L (1.09 mg/mL) for plasma oxalate, as determined based on data from 75 healthy adults.12
Per protocol, Cohort A patients who experienced progression of kidney impairment over time and required hemodialysis therapy crossed over to Cohort B. No patients crossed over in the 6-month primary analysis period. Two patients initiated hemodialysis in the extension period.13,15,16
Patients <10 kg received starting doses of 6.0 mg/kg monthly for 3 months and then ongoing doses of 3.0 mg/kg monthly; patients ≥10 to <20 kg received starting doses of 6.0 mg/kg monthly for 3 months and then ongoing doses of 6.0 mg/kg every 3 months; patients ≥20 kg received starting doses of 3.0 mg/kg monthly for 3 months and then ongoing doses of 3.0 mg/kg every 3 months.16
If administered on hemodialysis days, OXLUMO was administered after hemodialysis.1
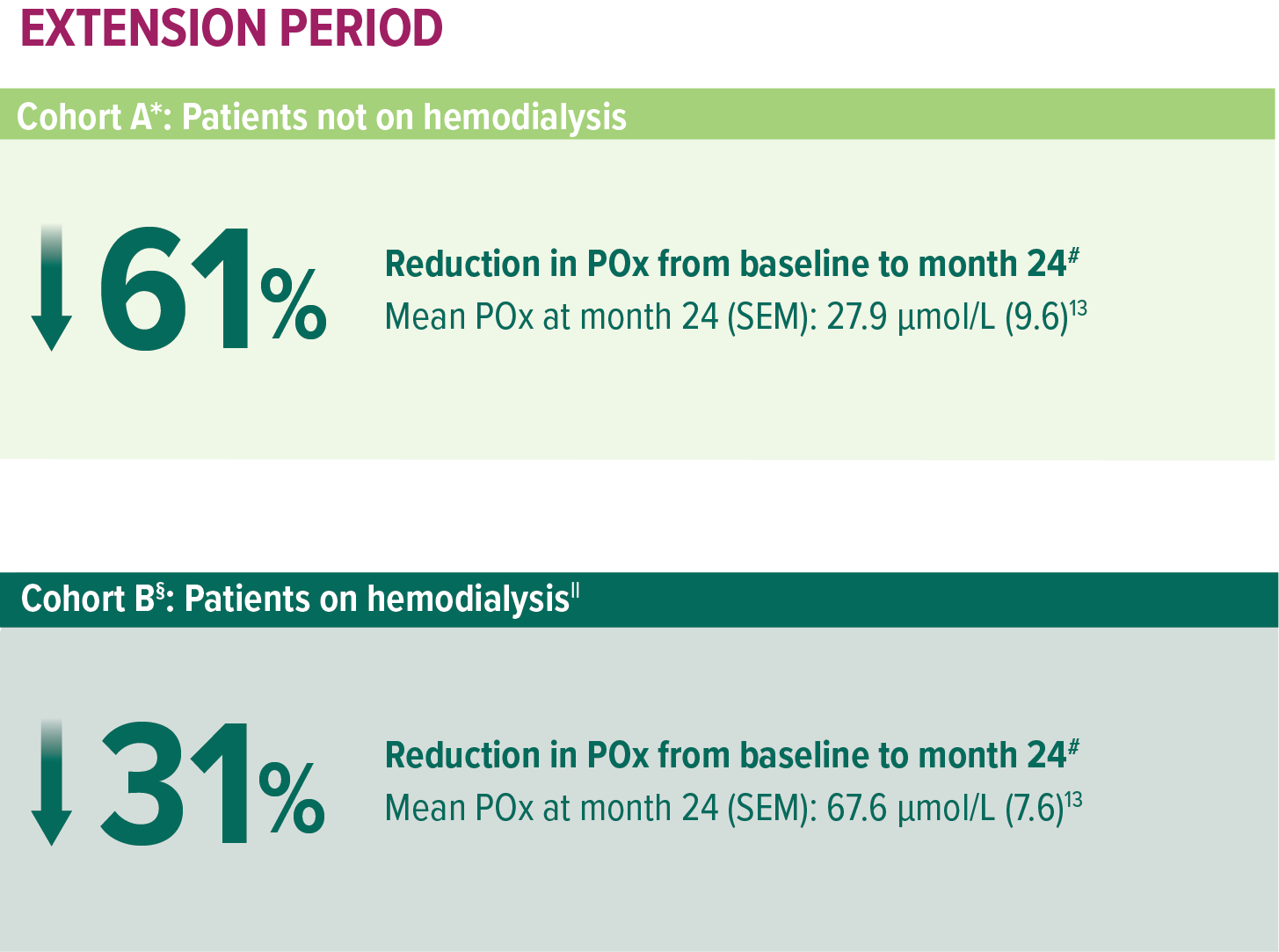
In ILLUMINATE-C, significant POx reduction in patients with advanced kidney disease, including those on hemodialysis1
Treatment with OXLUMO led to substantial POx reduction from baseline to 24 months in patients with advanced kidney disease and patients with kidney failure on hemodialysis (N=21)1,13
ILLUMINATE-C: Mean reduction in POx with OXLUMO1,13
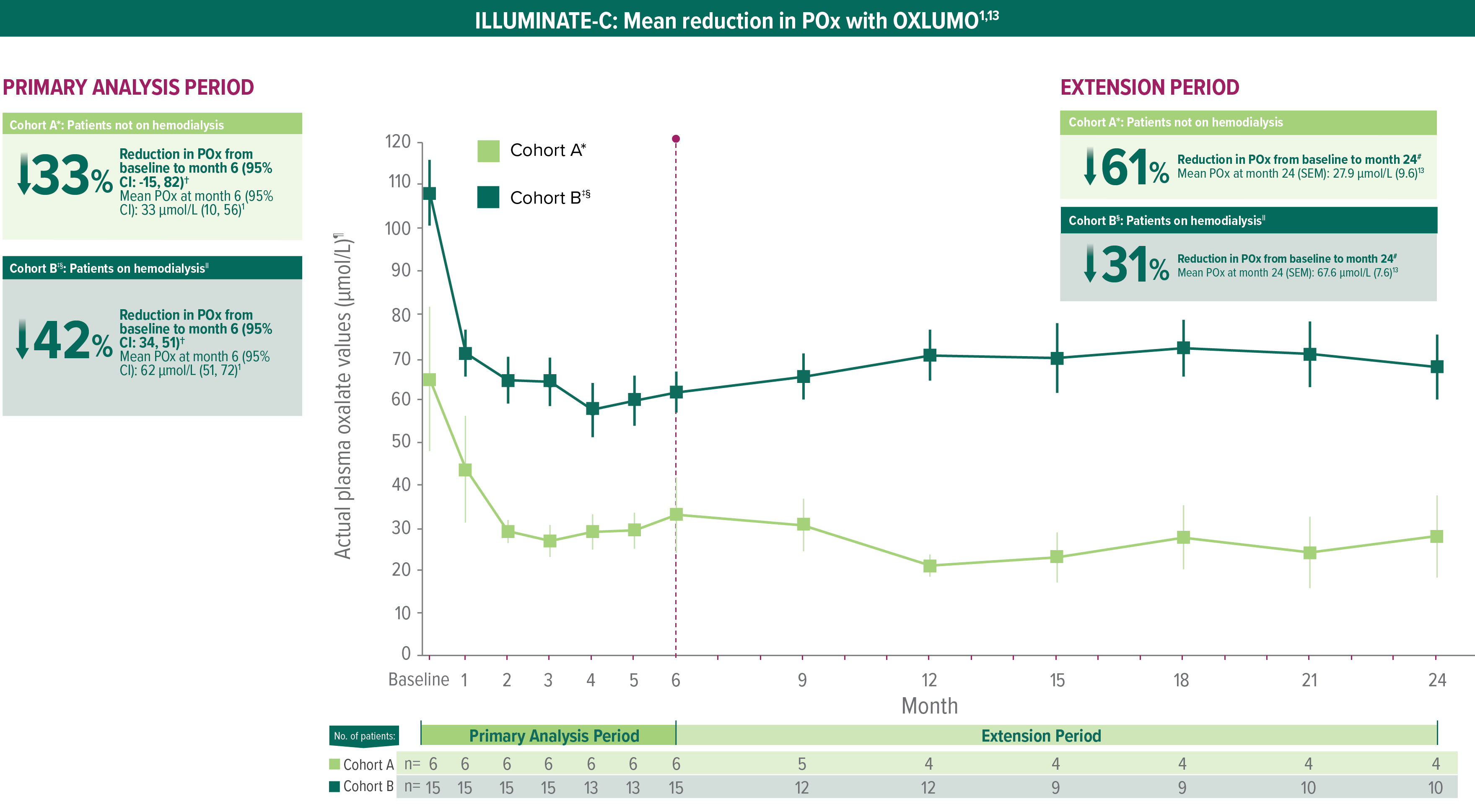
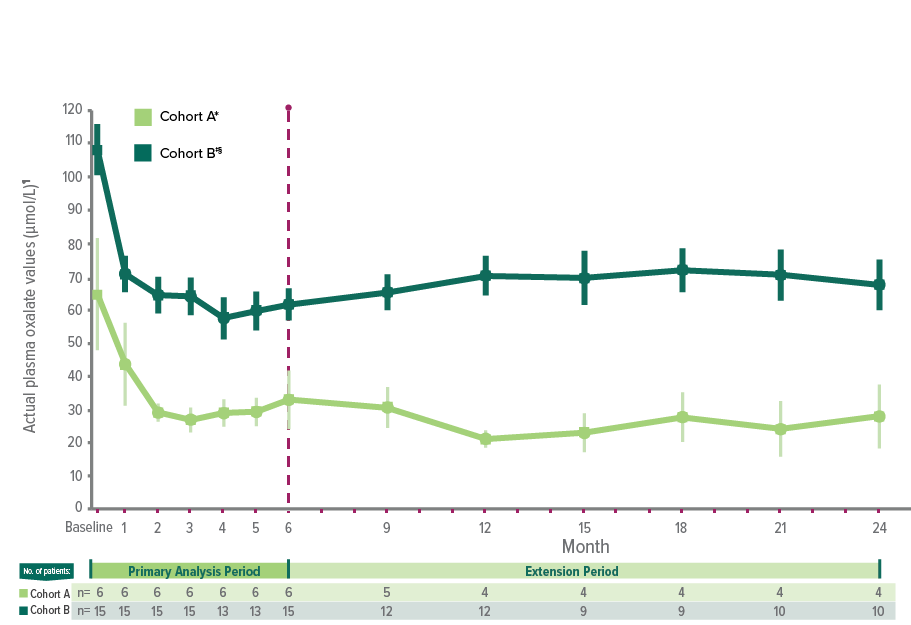
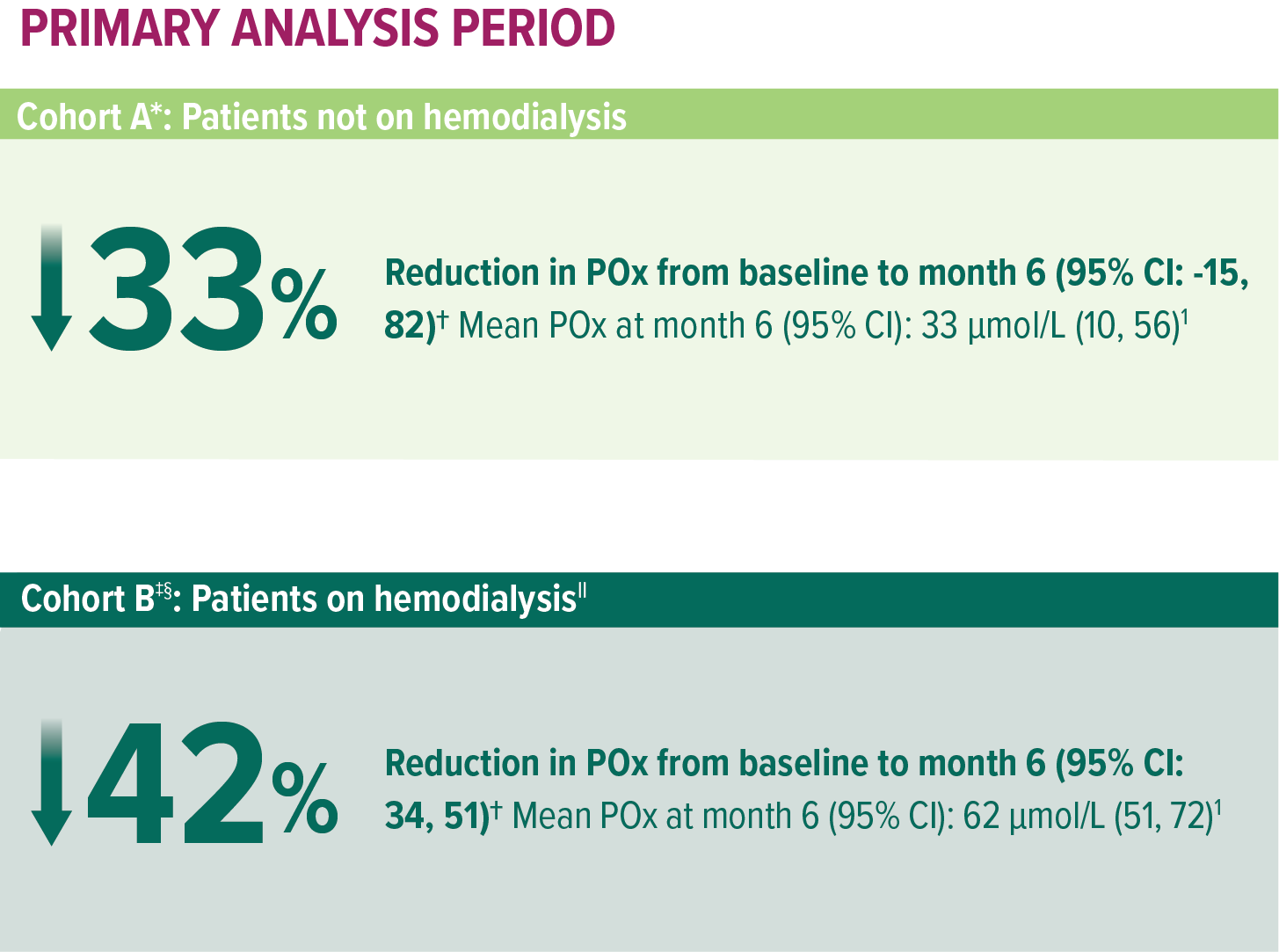
In Cohort A, baseline was defined as the mean of all POx samples (µmol/L) collected prior to the first dose of OXLUMO.13,15
The primary endpoint was based on the LS mean percent reduction from baseline to month 6 (which was calculated as an average of months 3 to 6).12,15
In Cohort B, baseline was defined as the mean of the last 4 predialysis POx samples (µmol/L) collected prior to the first dose of OXLUMO.13,15
Two patients moved from Cohort A to Cohort B.13
In Cohort B, POx was measured prior to a hemodialysis session.1
Mean and SEM actual POx values.13
POx mean percentage change from baseline to month 24.13
The safety profile of OXLUMO® (lumasiran) was evaluated in infants, children, and adults1
OXLUMO has been studied in 98 patients with PH1, including 71 pediatric patients and 15 patients on hemodialysis1
In placebo-controlled and open-label clinical studies the most common adverse reaction reported was injection site reaction. Injection site reactions included erythema, swelling, pain, hematoma, pruritus, and discoloration. These symptoms were generally mild and resolved within one day of the injection and did not lead to discontinuation of treatment.1
Safety during the 6-month double-blind period of ILLUMINATE-A
Adverse reactions reported in ≥10% of patients treated with OXLUMO and occurring ≥5% more frequently than in patients treated with placebo1
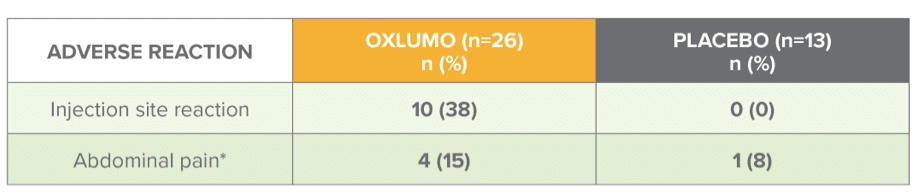
Grouped term includes abdominal pain, abdominal pain upper, abdominal pain lower, and abdominal discomfort.
During the extension period of ILLUMINATE-A (up to 36 months), the safety profile of OXLUMO was consistent with that seen in the double-blind period.1,4

WANT TO SPEAK WITH AN OXLUMO REPRESENTATIVE?
Our representatives are available to provide detailed information about OXLUMO and how it may help your patients with PH1.
There's more to learn about OXLUMO
When you sign up, we will keep you informed with updates and resources.
Alnylam Assist® provides support services for patients throughout their treatment with OXLUMO.
Alnylam Assist® includes patient services in 4 key areas, including understanding insurance benefits and financial assistance options for eligible patients,* helping ensure access to therapy, and providing PH1 disease education.
Patients must meet specified eligibility criteria to qualify for assistance. Alnylam reserves the right to make eligibility determinations and to modify or discontinue the program at any time.
1. OXLUMO Prescribing Information. Cambridge, MA: Alnylam Pharmaceuticals, Inc. 2. Garrelfs SF, Frishberg Y, Hulton SA, et al. N EngI J Med. 2021;384(13):1216-1226. 3. Hulton SA, Groothoff JW, Frishberg Y, et al. Kidney Int Rep. 2022;7(3):494-506. 4. Saland JM, Lieske JC, Groothoff JW, et al. Presented at National Kidney Foundation (NKF) Spring Clinical Meetings; April 11-15, 2023; Austin, TX. 5. Garrelfs SF, Frishberg Y, Hulton SA, et al. Supplementary appendix. N Engl J Med. 2021;384(13):1216-1226. 6. Data on file. Alnylam Pharmaceuticals, Inc. 7. Garrelfs SF, Frishberg Y, Hulton SA, et al. Protocol. N Engl J Med. 2021;384(13):1216-1226. 8. Sas DJ, Magen D, Hayes W, et al. Genet Med. 2022;24(3):654-662. 9. Alnylam Pharmaceuticals, Inc. Statistical Analysis Plan. August 2020. https://storage.googleapis.com/ctgov2-large docs/94/NCT03905694/SAP_001.pdf. Accessed August 5, 2024. 10. Michael M, Magen D, Hayes W, et al. Presented at the American Society of Pediatric Nephrology (ASPN) Meeting at PAS 2023; April 27-May 1, 2023; Washington, DC. 11. Hayes W, Sas DJ, Magen D, et al. Pediatr Nephrol. 2023;38(4):1075-1086. 12. Michael M, Groothoff JW, Shasha-Lavsky H, et al. Am J Kidney Dis. 2023;81(2):145-155. 13. Lieske J, Sellier-Leclerc A-L, Shasha-Lavsky H, et al. Poster presented at ASN Kidney Week; November 2-5, 2023; Philadelphia, PA. 14. Frishberg Y, Michael M, Groothoff JW, et al. Presented at: ASN Kidney Week; November 3-6, 2022; Orlando, FL, and Virtual. 15. Michael M, Groothoff JW, Shasha-Lavsky H, et al. Supplementary file 3. Statistical Analysis Plan. Am J Kidney Dis. 2023;81(2):145-155.1. https://www.ajkd.org/cms/10.1053/j.ajkd.2022.05.012/attachment/60c455e8-b3fb-47f7-812f-5af08a5fdd94/mmc3.pdf. 16. Michael M, Groothoff JW, Shasha-Lavsky H, et al. Supplementary File 1. Am J Kidney Dis. 2023;81(2):145-155.1.
